Table of Contents
Wednesday, March 16, 2016
Unit test!
Will update this with more information when I receive my score, but so far I thought it was fairly easy and hope that this trend will continue into further units.
Update: I did really well on this test! Probably my best test this year. Overall this unit wasn't too difficult and I hope to continue this trend in the future.
Chemical Bonding Unit Start!
This unit seems to be a continuation of the last unit. The good news is that last unit was relatively easy. I got a pretty good grade on the last unit too! So far, it seems to be easy, hopefully that will not change as we proceed into the future. We learned about finding valence and making Lewis dot diagrams. We also learned about how to measure bond length. This link will help with bond length: Bond length!
Chemical Bonding: Molecular Shapes!
 |
| New pictures! This one is of my dog, Fisher. |
1.) Tetrahedral
| http://people.uwplatt.edu/~sundin/images/vspr4.gif |
This molecule is non-polar with 4 bonds. It is 3D and has depth.
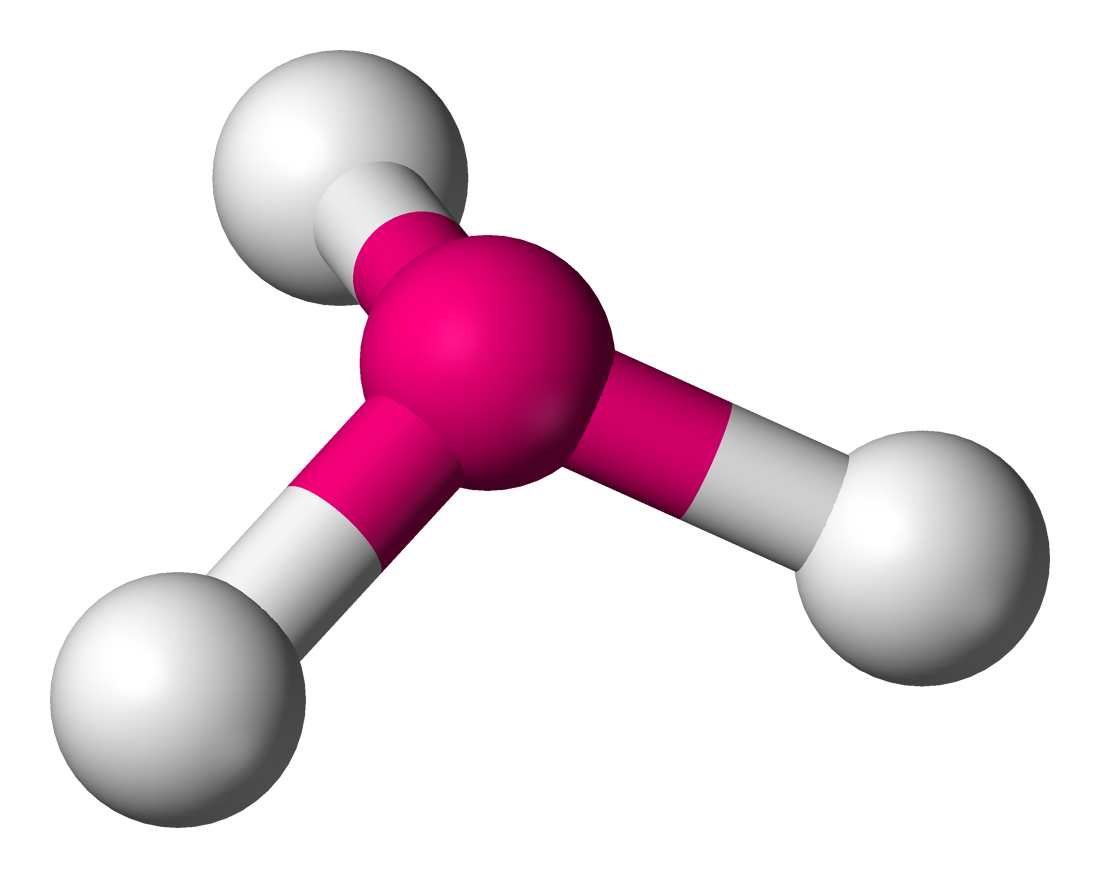 |
| https://upload.wikimedia.org/wikipedia/commons/e/e9/Pyramidal-3D-balls.png |
This molecule is polar and has 3 bonds with a lone pair on top.
3.) Trigonal Planar
| http://users.stlcc.edu/gkrishnan/vspr3.gif |
This molecule structure is like trigonal pyramidal, except that the lone pair on top is missing. This makes the molecule "2D". This molecule is non-polar.
4.) Linear
| http://www.ochempal.org/wp-content/images/D/dipolemoment9.png |
This molecule is also "2D" but it is locked in a straight line. This molecule is non-polar. Usually there will be on lone pairs on the central atom.
5.) Bent
| http://i.stack.imgur.com/xC0NU.jpg |
This molecule is polar, and usually has a pair of lone electrons on top of it. It has only two bonds.
6.) Octahedral
| http://chemwiki.ucdavis.edu/@api/deki/files/860/=copper(II)_fluoride.jpg?revision=1 |
This molecule is non-polar and has 6 bonds. It is three dimensional, and has depth.
More help on this topic can be found here: http://intro.chem.okstate.edu/1314f00/lecture/chapter10/vsepr.html
Sunday, March 6, 2016
Electronic Structure and Periodic Trends: TEST!
The test was taken, tears were shed. I wish that I had more time to study periodic trends. Maybe sooner, maybe later. My chem grade will update, but not with the A that I savor. Will update with more information as I receive it!
Electronic Structure and Periodic Trends: QUIZ!
This quiz was very easy for me and I hope it will signal a turning point for my chem grade. It's not as if my chem grade was in grave danger before this test, only that a nice boost was needed. This test mainly covered electron configuration and electron orbital rules. Hopefully this will be a good omen and the future lessons with be just as easily understood. For help on electron rules, click here.
Thursday, March 3, 2016
Electronic Structure and Periodic Trends: Electron Configuration!
We learned about how to make and read electron configurations. The first number tells you the principal energy level, the letter that comes after tells you the sublevel, and the superscript tells the number of electrons. For example, 1S² is Helium. If you need more help, you can click here! You can also make orbital level diagrams using this information. These can be useful to see if any of the 3 rules are being broken.
Electronic Structure and Periodic Trends: FLAME TEST LAB!
This lab was tons of fun and I wish we could do this one again! It was very cool to watch the different colors of fire from the different metals tested. This taught us about wavelengths of light and how it effects the energy of the effected metal. We put this information into a chart and then we had to convert the wavelength of light to energy in J/MOL. This was arguably the least fun part of the lab, but that's okay because it was still cool to see how the information we collected turns into numbers that we can use! To figure wavelength based on color, click here!
Electronic Structure and Peroidic Trends: LIGHT!
This unit starts off with a lesson about light! We learned about the differences between frequency and wavelength and how that effects the energy. The higher the frequency, and the smaller the wavelength, the more energy a photon has! We also learned to rank energy of light based on its name. For example, we know that Gamma rays have more energy than x-rays have more energy than ultra-violet light. We also learned that in the visible spectrum violet light is stronger than red light. To add to the information, we also were given a formula to figure out how much energy a photon of light has. The formula to figure out Frequency = C/W, and the formula to figure out Wavelength is C/F. Once you find the missing number, you can plug it into the energy equation: E = 6.63e-34(F).
If you need help figuring out energy, take a look here!
Subscribe to:
Comments (Atom)






