 |
| New pictures! This one is of my dog, Fisher. |
1.) Tetrahedral
| http://people.uwplatt.edu/~sundin/images/vspr4.gif |
This molecule is non-polar with 4 bonds. It is 3D and has depth.
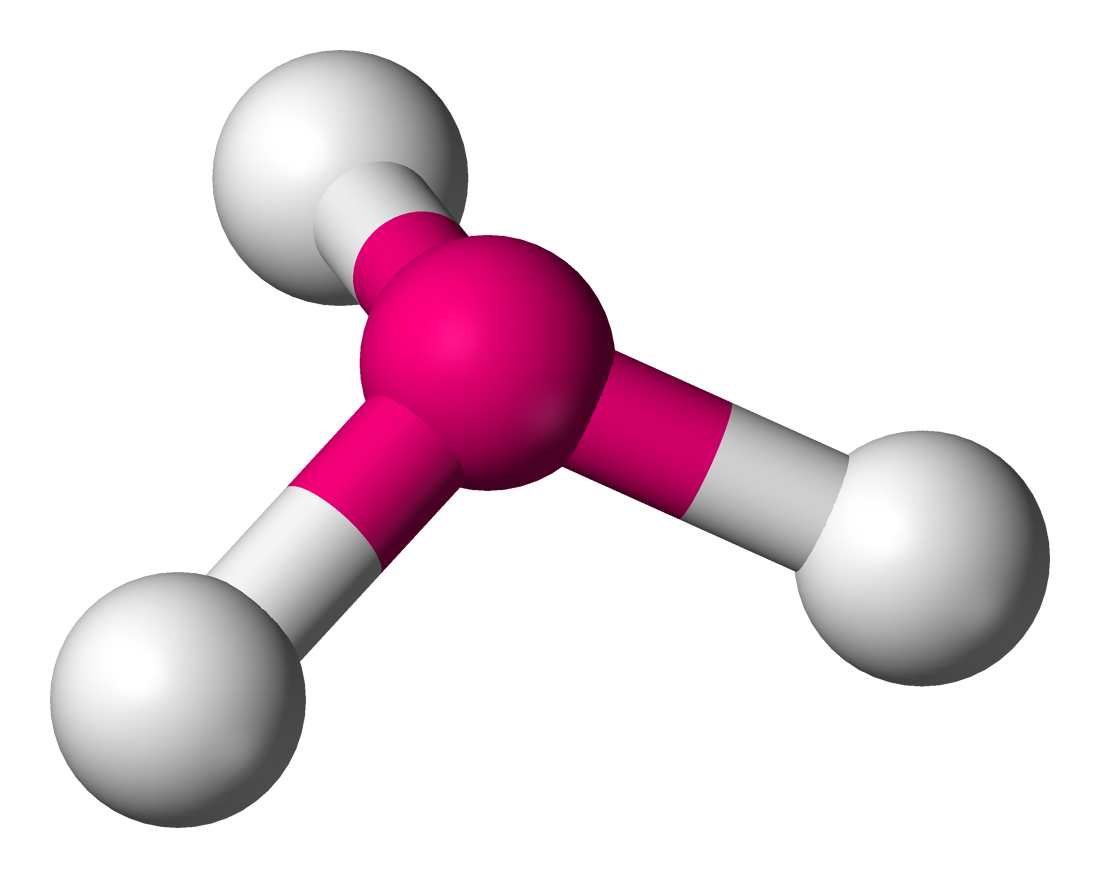 |
| https://upload.wikimedia.org/wikipedia/commons/e/e9/Pyramidal-3D-balls.png |
This molecule is polar and has 3 bonds with a lone pair on top.
3.) Trigonal Planar
| http://users.stlcc.edu/gkrishnan/vspr3.gif |
This molecule structure is like trigonal pyramidal, except that the lone pair on top is missing. This makes the molecule "2D". This molecule is non-polar.
4.) Linear
| http://www.ochempal.org/wp-content/images/D/dipolemoment9.png |
This molecule is also "2D" but it is locked in a straight line. This molecule is non-polar. Usually there will be on lone pairs on the central atom.
5.) Bent
| http://i.stack.imgur.com/xC0NU.jpg |
This molecule is polar, and usually has a pair of lone electrons on top of it. It has only two bonds.
6.) Octahedral
| http://chemwiki.ucdavis.edu/@api/deki/files/860/=copper(II)_fluoride.jpg?revision=1 |
This molecule is non-polar and has 6 bonds. It is three dimensional, and has depth.
More help on this topic can be found here: http://intro.chem.okstate.edu/1314f00/lecture/chapter10/vsepr.html
Thanks for posting this about just the molecular shapes. I found that memorizing the characteristics of these shapes was one of the hardest parts of this unit but this is a really nice summary of what we need to know! Also that's a really cool picture of your dog.
ReplyDelete