|
| Another Fisher Picture! |
Table of Contents
Monday, May 9, 2016
First Gas Lab! Airbag!
Gas Laws Quiz!
I am really scared to see how I have done on this quiz. The math I felt was easy, but I got completely mixed up by the concepts on it! I definitely need to go over this quiz with someone who knows more than I do. I just hope that it doesn't completely destroy my grade! I also want to make sure that I don't completely let the encroaching difficulty of this unit snowball my grade into oblivion. Another thing I am worried about getting tripped up on is converting units!
Gas Laws Combined Gas Theory.
This is the most interesting formula we have had since the original of PV/nT. (or as I say pressure and volume on a new table. Its kinda strange, I know, but its still helpful.) This lesson involved the use of PV/T = PV/T. This has the potential to get super complicated which is something I am always worried about. I am also scared about what we have coming at us in the future since we are only about halfway through this unit.
Gas Laws Unit Start!
 |
| Delicious Cupcakes! |
 We start off this unit nice and easy with Charles gas law. P1V1 = P2V2. This unit involves alot of math with equations and a lot of math with concepts to back them up. Hopefully this unit will not get too much harder when it comes to concepts so I won't struggle too much. I always fall behind on the abstract stuff when it comes to chem. Its hard to visualize floating invisible molecules and atoms sometimes.
We start off this unit nice and easy with Charles gas law. P1V1 = P2V2. This unit involves alot of math with equations and a lot of math with concepts to back them up. Hopefully this unit will not get too much harder when it comes to concepts so I won't struggle too much. I always fall behind on the abstract stuff when it comes to chem. Its hard to visualize floating invisible molecules and atoms sometimes.
Monday, May 2, 2016
Phase Changes and Heating / Cooling Curves!
 This section of the unit involved lots of graphs. The graphs that show us the supercritical point and when each of the phase changes occur and at what temperature and pressure. This allows us to predict phase changes and at what temperature they will occur and at what pressure. These graphs are called heating/cooling curves.
This section of the unit involved lots of graphs. The graphs that show us the supercritical point and when each of the phase changes occur and at what temperature and pressure. This allows us to predict phase changes and at what temperature they will occur and at what pressure. These graphs are called heating/cooling curves.Friday, April 29, 2016
Energy and Phase Changes Unit Begins!
 This unit begins with quite a mathy start. We start with learning about mcΔt, or mcat. This formula is used to figure out how much energy is during a phase change. You can also use the formula -mcΔt = mcΔt, which can be used to determine a missing variable from another equation. Luckily, this appears to be the hardest math in this unit, so hopefully everything else will be easy.
This unit begins with quite a mathy start. We start with learning about mcΔt, or mcat. This formula is used to figure out how much energy is during a phase change. You can also use the formula -mcΔt = mcΔt, which can be used to determine a missing variable from another equation. Luckily, this appears to be the hardest math in this unit, so hopefully everything else will be easy.
Boat Competition!
After we made our bio-diesel, the next part of the unit had us make boats to race! The boats were made out of all sorts of things, like cardboard and plastic. We made ours out of a butter/lard container. The sides of the boat were high and it was pretty light. Our team did have some trouble at first trying to get it to work, but eventually we managed to fix all the issues just in time for the race! We came in second place!
How to make a putt putt boat: http://www.sciencetoymaker.org/boat/asembCartonl.html
How to make biodiesel: http://www.make-biodiesel.org/
How to use biodiesel: http://biodiesel.org/using-biodiesel/fuel-quality-guide
Biodiesel Construction!
 We made bio diesel in a recent lab! It wasn't the most fun experience I have ever had, but it was still interesting to see how it was made. We placed depleted cooking into a beaker with a strong base, and then we used a spinner to spin the mixture for about 20 minutes. After that, our bio-diesel was ready to sit! One day later, the bio-diesel was ready, we just had to extract it from the cup. How to make biodiesel: http://www.make-biodiesel.org/
We made bio diesel in a recent lab! It wasn't the most fun experience I have ever had, but it was still interesting to see how it was made. We placed depleted cooking into a beaker with a strong base, and then we used a spinner to spin the mixture for about 20 minutes. After that, our bio-diesel was ready to sit! One day later, the bio-diesel was ready, we just had to extract it from the cup. How to make biodiesel: http://www.make-biodiesel.org/What is biodiesel? http://biodiesel.org/what-is-biodiesel/biodiesel-basics
Wednesday, April 6, 2016
Biodiesel Contest Video!
This is the final copy of the biodiesel video Lindsi and I made for the ALAUM high school contest! Enjoy!
Link to the rest of the videos: https://www.youtube.com/user/ALAUMEnvironment
Wednesday, March 16, 2016
Unit test!
Will update this with more information when I receive my score, but so far I thought it was fairly easy and hope that this trend will continue into further units.
Update: I did really well on this test! Probably my best test this year. Overall this unit wasn't too difficult and I hope to continue this trend in the future.
Chemical Bonding Unit Start!
This unit seems to be a continuation of the last unit. The good news is that last unit was relatively easy. I got a pretty good grade on the last unit too! So far, it seems to be easy, hopefully that will not change as we proceed into the future. We learned about finding valence and making Lewis dot diagrams. We also learned about how to measure bond length. This link will help with bond length: Bond length!
Chemical Bonding: Molecular Shapes!
 |
| New pictures! This one is of my dog, Fisher. |
1.) Tetrahedral
| http://people.uwplatt.edu/~sundin/images/vspr4.gif |
This molecule is non-polar with 4 bonds. It is 3D and has depth.
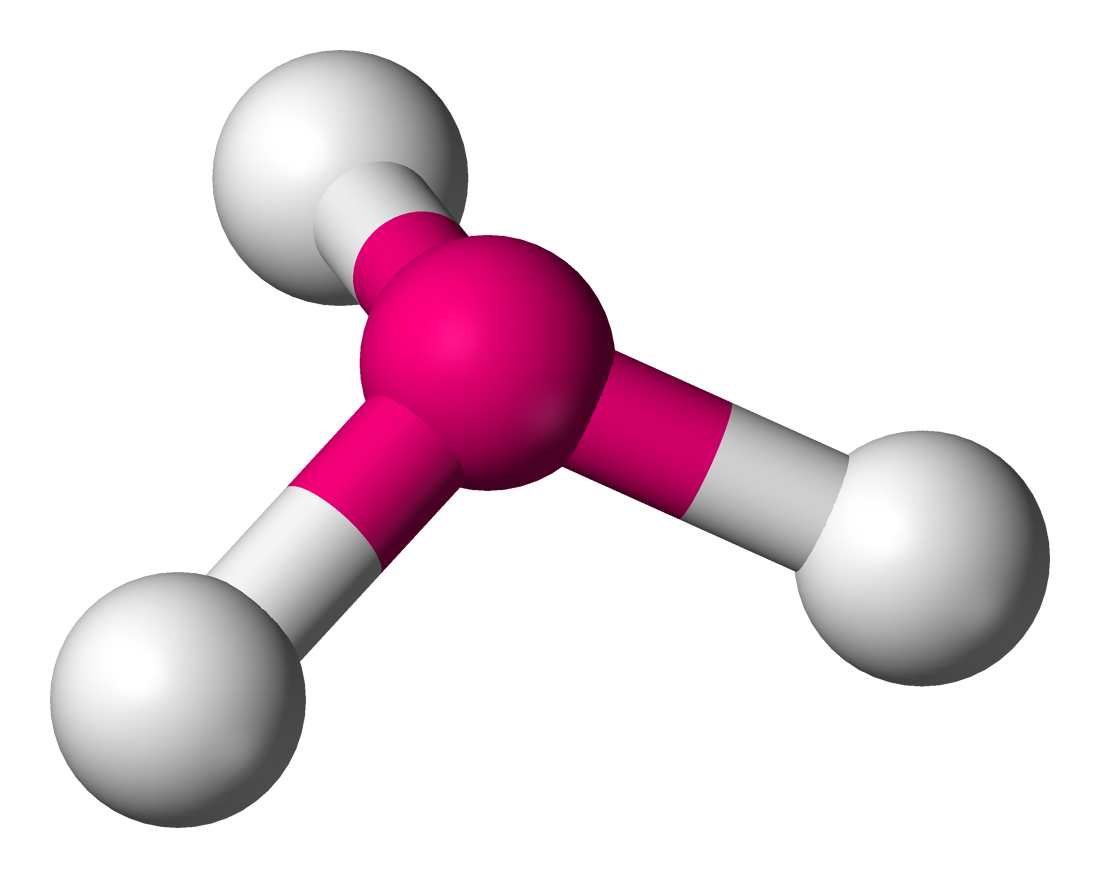 |
| https://upload.wikimedia.org/wikipedia/commons/e/e9/Pyramidal-3D-balls.png |
This molecule is polar and has 3 bonds with a lone pair on top.
3.) Trigonal Planar
| http://users.stlcc.edu/gkrishnan/vspr3.gif |
This molecule structure is like trigonal pyramidal, except that the lone pair on top is missing. This makes the molecule "2D". This molecule is non-polar.
4.) Linear
| http://www.ochempal.org/wp-content/images/D/dipolemoment9.png |
This molecule is also "2D" but it is locked in a straight line. This molecule is non-polar. Usually there will be on lone pairs on the central atom.
5.) Bent
| http://i.stack.imgur.com/xC0NU.jpg |
This molecule is polar, and usually has a pair of lone electrons on top of it. It has only two bonds.
6.) Octahedral
| http://chemwiki.ucdavis.edu/@api/deki/files/860/=copper(II)_fluoride.jpg?revision=1 |
This molecule is non-polar and has 6 bonds. It is three dimensional, and has depth.
More help on this topic can be found here: http://intro.chem.okstate.edu/1314f00/lecture/chapter10/vsepr.html
Sunday, March 6, 2016
Electronic Structure and Periodic Trends: TEST!
The test was taken, tears were shed. I wish that I had more time to study periodic trends. Maybe sooner, maybe later. My chem grade will update, but not with the A that I savor. Will update with more information as I receive it!
Electronic Structure and Periodic Trends: QUIZ!
This quiz was very easy for me and I hope it will signal a turning point for my chem grade. It's not as if my chem grade was in grave danger before this test, only that a nice boost was needed. This test mainly covered electron configuration and electron orbital rules. Hopefully this will be a good omen and the future lessons with be just as easily understood. For help on electron rules, click here.
Thursday, March 3, 2016
Electronic Structure and Periodic Trends: Electron Configuration!
We learned about how to make and read electron configurations. The first number tells you the principal energy level, the letter that comes after tells you the sublevel, and the superscript tells the number of electrons. For example, 1S² is Helium. If you need more help, you can click here! You can also make orbital level diagrams using this information. These can be useful to see if any of the 3 rules are being broken.
Electronic Structure and Periodic Trends: FLAME TEST LAB!
This lab was tons of fun and I wish we could do this one again! It was very cool to watch the different colors of fire from the different metals tested. This taught us about wavelengths of light and how it effects the energy of the effected metal. We put this information into a chart and then we had to convert the wavelength of light to energy in J/MOL. This was arguably the least fun part of the lab, but that's okay because it was still cool to see how the information we collected turns into numbers that we can use! To figure wavelength based on color, click here!
Electronic Structure and Peroidic Trends: LIGHT!
This unit starts off with a lesson about light! We learned about the differences between frequency and wavelength and how that effects the energy. The higher the frequency, and the smaller the wavelength, the more energy a photon has! We also learned to rank energy of light based on its name. For example, we know that Gamma rays have more energy than x-rays have more energy than ultra-violet light. We also learned that in the visible spectrum violet light is stronger than red light. To add to the information, we also were given a formula to figure out how much energy a photon of light has. The formula to figure out Frequency = C/W, and the formula to figure out Wavelength is C/F. Once you find the missing number, you can plug it into the energy equation: E = 6.63e-34(F).
If you need help figuring out energy, take a look here!
Tuesday, February 16, 2016
New Unit! Electronic Structure and Periodic Trends!
Electronic Structure and Periodic Trends
We start this new unit off easy with an article and worksheet on fireworks. I'm not sure how this will tie into the unit, as mainly I am not quite sure what this unit is about. I am interested to find out more though. All I can hope for is that this is a easier unit that the Acids and Bases unit. I guess this is early reading for the unit: ChemWiki Periodic Trends
We start this new unit off easy with an article and worksheet on fireworks. I'm not sure how this will tie into the unit, as mainly I am not quite sure what this unit is about. I am interested to find out more though. All I can hope for is that this is a easier unit that the Acids and Bases unit. I guess this is early reading for the unit: ChemWiki Periodic Trends
Acids and Bases Unit Test Follow-up.
Unit Follow-up
This unit was one on the most difficult one of the year. Hopefully I didn't do too bad on the test, as that would definitely hamper my grade. Although I don't feel I did too bad. I will update this post when I get the results.
UPDATE: I didn't do horribly on this test, but I could have done better and I wish I would have studied how to calculate titrations more. It didn't effect my grade too much, so I hope that I the next unit test will not go as bad.
 |
| Weird Photo. Don't know what happened to it. |
UPDATE: I didn't do horribly on this test, but I could have done better and I wish I would have studied how to calculate titrations more. It didn't effect my grade too much, so I hope that I the next unit test will not go as bad.
Wednesday, February 10, 2016
UNIT TEST! ACIDS AND BASES
The Unit Test
The unit test is tomorrow. This is very scary. I need to do well on this test, but I always freeze up and can't do it. Hopefully this time will be different. I will do double the studying and double the work. Will update this when the test grade returns.
Resources:How to Find pH and pOH. How to find how many times more acidic or basic something is.
Acids and Bases - Second Lab
Second Lab
This second lab we did was very similar to the first, but instead of finding the moles of acetic acid, we were trying to find the molar mass of an unknown acid. The process of titrating the unknown acid was relatively the same process. However, there was some unforeseen complications. The unknown acid did not dissolve easily. The secret ingredient was heat, but the heat will evaporate the pHen required to titrate. So it was a lose - lose. However, we managed to get the lab done and get a decent grade. Titration help:http://www.westfield.ma.edu/cmasi/gen_chem1/Solutions/reactions%20in%20solution/solution%20stoichiometry/titration.htm
Acids and Bases - FIRST LAB!
First Lab
The first lab we have done in this unit is the Percent Acetic Acid in Vinegar. This lab was really amazing because of the freedom we got while competing it. Basically, all the materials required to complete the lab were laid out on a middle table and the tools required to complete the lab were at the station, then we got three days to do whatever we need to do to finish the lab. We finished the lab by titrating the acid and standardizing the base. Titration Resources: https://www.dartmouth.edu/~chemlab/techniques/titration.html
Acids and Bases Weekly Quiz
Acids and Bases Weekly Quiz
I thought I knew alot on the quiz, but I guess I was wrong. I didn't get a fantastic grade on the quiz and it wasn't for the reasons I thought. I expected to get the ICE Box questions wrong but I got most of those right. Unfortunately for me, I didn't know how to graph ice box questions when I took this, so I had alot of trouble. I also didn't know how to figure out how many more times more acidic or basic something was. I have since learned and I can assure myself that I will not get those wrong on the test! Resources for ICE Box: http://www.chem.purdue.edu/gchelp/howtosolveit/Equilibrium/ICEchart.htm
Acids and Bases
Acids and Bases
I am excited for this Unit riding off an okay grade for the Aqueous Solutions test. In the first couple of days we learned about the differences between arrhenius acids and bases and Brønsted–Lowry acids and bases. We learned the properties of these acids and what their equations would look like. A helpful resource for this is: http://www.chemguide.co.uk/physical/acidbaseeqia/theories.html
I am excited for this Unit riding off an okay grade for the Aqueous Solutions test. In the first couple of days we learned about the differences between arrhenius acids and bases and Brønsted–Lowry acids and bases. We learned the properties of these acids and what their equations would look like. A helpful resource for this is: http://www.chemguide.co.uk/physical/acidbaseeqia/theories.html
Monday, January 25, 2016
Aqueous Solutions Test!
The test wasn't very complicated, and I feel as if I did good on it. I believe I got an %86 on it, so I feel pretty good. Hopefully this will be a omen for the units to come, as I would really like to get close to an A. Hopefully with the new study techniques I've developed, I can do better on these tests in the future.
Friday, January 15, 2016
Aqueous Solutions Weekly Test
WEEKLY QUIZ
Murder Mystery Lab
MURDER MYSTERY
The murder mystery lab is one that is fun to complete. Even after some difficult math and chemical predictions, it was still cool to see that come to fruition and watch as the chemical reaction unfolds just as you predicted it would, and bringing your self one step closer to solving the murder. This lab is much more involved than ones previous, making it a bit strange and confusing at times. I would get confused on what to do next even though we had a basic procedure written. Something cool about this lab was that we also got to choose exactly how we ran it! If you want to use well plates, you can, but you don't have to. This made the lab an experience much like I would expect a real laboratory to be.
Thursday, January 7, 2016
Aqueous Solutions DAY 1!
Aqueous Solutions
Today we began a brand new pretty confusing unit! That is to be expected though. This unit, as evidenced by the title of this post, is about aqueous solutions! However, there is a bit more baggage that comes along with this unit besides just aqueous solutions. I didn't realize how much substance would be in this unit. It's not that it is too difficult, it's that there is a lot to take in and comprehend.
The general principle of 'convert to moles' hasn't changed. Which for the most part is a very good thing. I hope that after some reading and studying I will have a better grasp on the concepts being presented in this unit!
Today we began a brand new pretty confusing unit! That is to be expected though. This unit, as evidenced by the title of this post, is about aqueous solutions! However, there is a bit more baggage that comes along with this unit besides just aqueous solutions. I didn't realize how much substance would be in this unit. It's not that it is too difficult, it's that there is a lot to take in and comprehend.
The general principle of 'convert to moles' hasn't changed. Which for the most part is a very good thing. I hope that after some reading and studying I will have a better grasp on the concepts being presented in this unit!
Subscribe to:
Comments (Atom)